August 2017 Newsletter
Worried About Fake News? You Should Really Worry about Fake Drugs
By Sarah Keefe & Stephen Shaw
Womble Carlyle Sandridge & Rice, LLP
While the concept of “fake news” continues to trigger Twitter followers and grab headlines, the trade in counterfeit drugs is a worldwide problem of significant scale. This article discusses the problem including what trademark owners are doing to address the public safety issues posed by fake drugs.
The Problem
Trademark counterfeiting? Most think of fake Rolex watches sold on city street corners or faux designer purses sold in suburban kitchens. While knock-off luxury goods may seem harmless, the economic harm inflicted on legitimate businesses—and the workers they employ—is enormous. The Federal Bureau of Investigation estimates that the dollar value of counterfeit goods sold in the US at $200-250 billion annually. Many of North Carolina’s leading industries—including tobacco, furniture, apparel, and pharmaceuticals—are among the most frequent targets of trademark counterfeiting. Also, according to Interpol, trademark counterfeiting often provides for a source of income and a reliable import channel for those with other nefarious purposes such as illegal drug or human trafficking and potentially even terrorist activities.
In addition to these very negative economic consequences, there is a particular level of harm associated with certain kinds of trademark counterfeiting. Counterfeit goods aren’t subject to the same regulatory standards and safety inspections as items produced by legitimate manufacturers. So, in areas of manufacture where quality control is important, counterfeit products fall short. Consider, for example, the potential harm that could be caused by substandard, bogus automobile tires or airplane parts.
Counterfeit pharmaceutical drugs are clearly an area where public safety concerns are paramount. Counterfeit medicines have been found in all areas of the globe, including the highly regulated US market, and extend to medicines, vaccines, testing and diagnostic equipment, and other medical devices. Both branded and generic pharmaceutical products can be falsified. And sales are not just limited to the black market—such products make their way to hospitals and pharmacies, often through internet trade channels.
Lifestyle or “popular” drugs are often counterfeited, and there have been recent increases in cosmetic, weight loss, and opioid drugs. However, counterfeit malaria vaccines, cholesterol medications, and cancer treatments can also be found in the US. The World Health Organization (WHO) estimates that an average of up to 10% of medicines are counterfeit, and in developing countries, that percentage is considerably higher.
The Center for Medicine in the Public Interest estimates the annual worldwide dollar value of counterfeit pharmaceuticals at $200 billion annually and rising. Sadly, the damage is far greater than lost sales. WHO estimates that approximately 200,000 people die each year as the result of ineffective counterfeit anti-malarial drugs.
Counterfeit medicines also may cause adverse effects or allergic reactions, and they may not effectively treat the ailment for which they were prescribed. They may promote drug resistant disease strains or contain no active ingredient, the wrong active ingredient, or the incorrect strength (too much or too little) or dosage of the intended ingredient. According to a WHO investigation, approximately 1/3 of counterfeit drugs contain no effective ingredient. Given the lack of inspection of counterfeit manufacturing facilities, the drugs are often produced under non-sterile circumstances, resulting in bacterial or other contamination. Clearly, the ways in which counterfeit drugs can cause serious negative patient outcomes are extensive and extreme.
Combating the Problem
Legitimate drug companies, and their attorneys and security departments, take a multi-pronged approach to combating counterfeiting. Many of these efforts are used to combat counterfeiting generally, but some have been developed with particular focus toward the nuances of the counterfeit pharmaceutical market.
The counterfeiting problem can appear daunting, given the array of unknown manufacturing sources, the breadth of possible sales outlets, and the strong consumer preference for the “cheap but name brand” combination. Successful anti-counterfeiting practices do not generally follow a reactive “Whack-A-Mole” approach, attempting to squelch every low-level advertisement or email blast that comes out. Rather, systematic and strategic prosecution, akin to practices used in the intelligence community, is preferred. Investigations of networks and relationships, rather than merely products and sellers, can help identify high-payoff targets, the disruption of which can have positive effects across several echelons of the counterfeit trade. Focusing limited enforcement resources on high-payoff choke points can impact not just the enforcement subject, but others in the counterfeiting ecosystem, which is a fruitful and strategic approach.
Counterfeit pharmaceuticals in particular, given their specific methods of typical advertisement and sale, are susceptible to certain forms of interdiction over others. It is well known that counterfeit pharmaceuticals are advertised heavily on both internet ads and email “spam” messages, all of which attempt to direct a prospective purchaser to a product-ordering website. Attempting to impede the advertising messages, which are often sent by botnets or third-parties through a referral-affiliate relationship, or to shut down specific websites, is a losing proposition because there are hundreds of thousands or more of each, and the costs to re-establish a confiscated website are negligible compared to the significant enforcement costs. However, academic researchers working with industry and enforcement contacts identified several potential chokepoints on which these sorts of pharmaceutical sales pathways rely. See Dharmdasani et al., “Priceless: The Role of Payments in Abuse-advertised Goods,” CCS ’12, October 16–18, 2012, Raleigh, NC. One key chokepoint relationship for online transactions is a seller’s host bank, nearly always non-US and non-EU, which is willing to accept abusive credit card transactions related to these unlawful goods from card processors such as Visa and MasterCard.
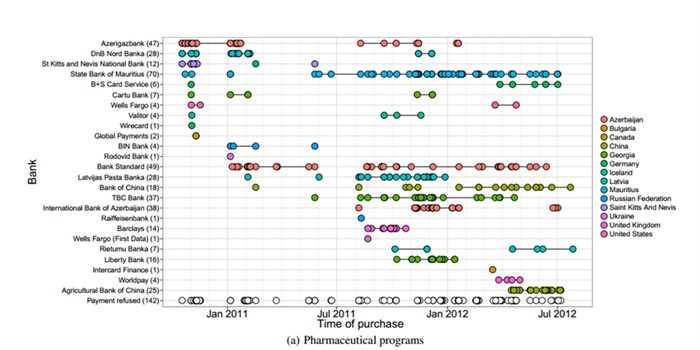
Image credit: Damon McCoy, Hitesh Dharmdasani (George Mason University); Christian Kreibich (University of California, San Diego and International Computer Science Institute); and Geoffrey M. Voelker and Stefan Savage (University of California, San Diego)
Nearly all online counterfeit pharmaceutical revenue can flow through these concentrated banking relationships, and researchers discovered that intercepting just one or two of these banking relationships, and seizing related funds, could have ripple effects disrupting thousands of drug transaction websites and referral affiliates. For example, a 2011 study determined that across 95% of spam email for pharmaceuticals and similar goods, only three acquiring bank institutions were used. Id. This research demonstrates a guiding principal of anti-counterfeiting efforts: enforcement is most successful where the “termination cost [to the counterfeiter] is inevitably far higher— in fines, in lost holdback, in time, and in opportunity cost—than the cost of the intervention itself [by the rights holder]. … [R]elatively concentrated actions with key . . . institutions can have outsized impacts.” Id.
Targeted investigations and enforcement are only one aspect of a comprehensive anti-counterfeiting approach. Successful rights holders employ a broad-based strategy, involving secured supply chain management, thorough technology and distribution agreements with market partners, and high-tech security solutions such as secure packaging, microchips, holograms, and the like. The combination of many of these controls can make enforcement actions easier and more productive, and thus reduce the prevalence and success of counterfeit competitors. In addition, it is important to inform consumers and retailers about both the dangers of counterfeits and the value of accessing authentic products. Pharmaceutical companies should engage in advertising to alert the marketplace to the dangers of counterfeit pharmaceuticals and to also assist others in identifying counterfeit drugs—including examination of packaging and medicines for quality, condition, spelling, and grammar; and checking manufacturing and expiration dates and batch numbers on both exterior and interior packaging.
The problem of fake drugs is in fact very real news, and companies here in North Carolina and around the world are taking steps to defend their brands and protect their customers in the marketplace.
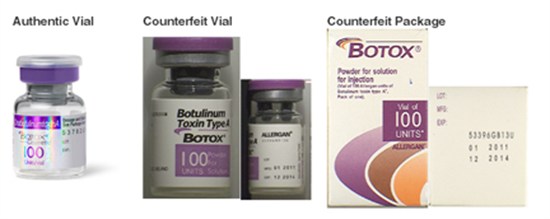
April 16, 2015 FDA alert.
Sarah Keefe leads Womble Carlyle’s Trademarks, Copyrights, and Transactions Practice Group. She is a North Carolina board certified specialist in trademark law. Her particular experience is in pharmaceutical trademarks, and developing and efficiently maintaining worldwide intellectual property portfolios.
Stephen Shaw is an anti-counterfeiting and brand protection litigator who loves chasing knock-offs for strategic business effects. His experience includes litigation of major intellectual property issues in a breadth of domains, including anti-counterfeiting as well as copyright, trademark, and patent disputes. Stephen has experience with clearance and counseling related to a variety of IP protection methods, and he often assists rights holders in tailoring their IP regimes to the most efficient effect against infringers.
Spotlight: Social Security Attorney John Koontz
John Koontz is a social security disability law specialist with Daggett Shuler. We asked him seven questions about his work and the challenges he faces representing the disabled.
1. What is most challenging about your work?
 I think there are two big challenges to handling disability cases. The first is the interplay or juncture of law and medicine. You need a good working knowledge of medicine so that you can understand how your client’s physical and mental conditions impact their functional abilities. At the same time, you have to understand how those functional limitations fit within the extensive legal framework for disability claims. This means you have to understand and apply the appropriate statutes (Social Security Act), federal regulations, rulings from the Social Security Administration, and decisions from the federal courts. The second major challenge is managing the client’s expectations. Every client believes they are disabled and that the Social Security Administration should approve their claim. Unfortunately, it is not always that simple or straight-forward. As the claims process grinds on over months and even years, the stress on the client increases as their financial situation erodes and their health deteriorates. They may become desperate or even hopeless, and it can be very challenging to manage their expectations.
I think there are two big challenges to handling disability cases. The first is the interplay or juncture of law and medicine. You need a good working knowledge of medicine so that you can understand how your client’s physical and mental conditions impact their functional abilities. At the same time, you have to understand how those functional limitations fit within the extensive legal framework for disability claims. This means you have to understand and apply the appropriate statutes (Social Security Act), federal regulations, rulings from the Social Security Administration, and decisions from the federal courts. The second major challenge is managing the client’s expectations. Every client believes they are disabled and that the Social Security Administration should approve their claim. Unfortunately, it is not always that simple or straight-forward. As the claims process grinds on over months and even years, the stress on the client increases as their financial situation erodes and their health deteriorates. They may become desperate or even hopeless, and it can be very challenging to manage their expectations.
2. What is most fun about your work?
From a practical standpoint, I really enjoy the medical component of each case. It is fascinating to me to go through the medical records and piece together each client’s medical conditions, and discover how those medical conditions interfere with or affect their ability to do things. Doing this brings a great deal of medicine into play, and I find that I am constantly learning something new, which I enjoy. From a personal standpoint, I really like helping our clients get back on their feet. Most of them have lost so much, not the least of which is their health, and they desperately need someone to help them. It is very rewarding to work with them on such a long journey and see them get the disability benefits that they deserve. This is often a life-changing moment for them and their families and it is very rewarding to be a part of that.
3. What led you to become a lawyer?
My father is an attorney in Concord, so I grew up in the legal community around attorneys and judges. Then I married my high school sweetheart, and her father is an attorney. So, law school was always something that was in the back of my mind. However, my original plan was to get my doctorate in political science and work for the government or teach at a university. After I got my master’s degree at the University of Georgia, I decided that I wanted to have more career options and flexibility with where I lived and raised my family. I thought law school would do that, so my wife and I with our new daughter moved to Knoxville and I attended law school at the University of Tennessee. Obviously, this means we are conflicted each year when Georgia and Tennessee meet on the gridiron.
4. What made you decide to pursue certification?
I believe certification is a measure or indicator of an attorney’s dedication to a practice area and the level at which it is being practiced. This is because getting certified and maintaining certification is a rigorous process. To be certified you have to pass an examination that measures your competency in the practice area, you have to show that you devote a significant number of hours to the practice area, you must attend a specific number of CLE hours that are related to the practice area, and you have to receive favorable reviews from your peers. Recertification involves the same requirements except that you do not have to pass another exam. As a result, recertification confirms that you remain committed to the practice area and that you are practicing it at the highest level. Like most of us, my parents instilled in me the expectation that I would always strive to do my best at whatever endeavor I was pursuing. Since certification represents the highest level of devotion and achievement in my practice area, I naturally wanted to be certified as a specialist when it became available through the State Bar.
5. What is it like to work with clients who are managing disabilities in their lives?
It can be very challenging because representing clients in disability claims goes well beyond just the case. At some point, almost all disability clients have additional issues that must be addressed. For example, they may not have health insurance or the financial resources to get the medical treatment they need, so you have to find them free or reduced fee clinics. They may be facing foreclosure, eviction, or repossession notices due to their loss of income, so you have to steer them to legal resources that can help them with these problems. They may have mental health or substance abuse problems that are not being addressed, so you have to get them to mental health facilities. Finally, they may not be able to put food on the table or keep their lights on, so you have to get them in touch with community organizations that can help them with these financial needs. It really involves a holistic approach to helping the client, with the disability claim being just one of their needs.
6. What activities/volunteer groups are you involved in?
I am active with the National Organization of Social Security Claimants’ Representatives (NOSSCR) and the North Carolina Advocates for Justice. I have spoken at disability CLEs in the past and before community groups about various disability topics. The firm is very active in the community and sponsors many events throughout the year. Our biggest event is the Safe and Sober Prom Night Program. This lasts for several months during prom season and I, as well as the other attorneys and staff at the firm, take turns visiting more than 40 area high schools to put on inspirational programs and challenge the students to remain safe and sober on prom night. It is a major undertaking for the firm, but we are all dedicated to this message.
7. Who is your role model and why?
The other attorneys at Daggett Shuler are my role models. I know that may sound a little self-serving, but it is the truth. It is a pleasure to work with them and they are an inspiration to me. I have the utmost respect for each one of them as attorneys as well as individuals of integrity. They set the highest standards of professionalism and competency in their practice areas, and the highest standards of integrity and morality as individuals. That shared culture is probably why we have practiced together for so long. I know that I can count on them to support and encourage me in my law practice as well as in my personal life.
Specialist Notes: Time for Recertification
Please remember that specialists up for recertification in 2017 must submit a recertification application before September 8, 2017, to avoid being assessed a $250 late application fee. If you have questions about your recertification, please contact Lanice Heidbrink at 919-719-9271 or by email lheidbrink@ncbar.gov.